
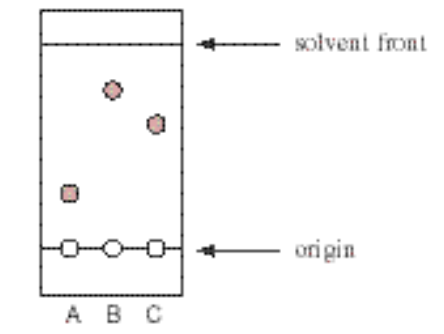
Consider the following silica gel TLC plate of compounds A, B, and C developed in hexane and answer the following questions. a) Determine the Rf values of compounds A, B, and C (
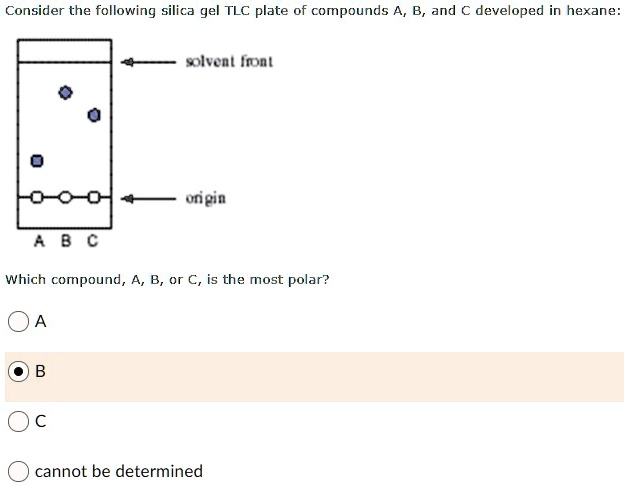
SOLVED: Consider the following silica gel TLC plate of compounds A and C developed in hexane: slvent Imnt npin Which compound; A, or C, is the most polar? cannot be determined
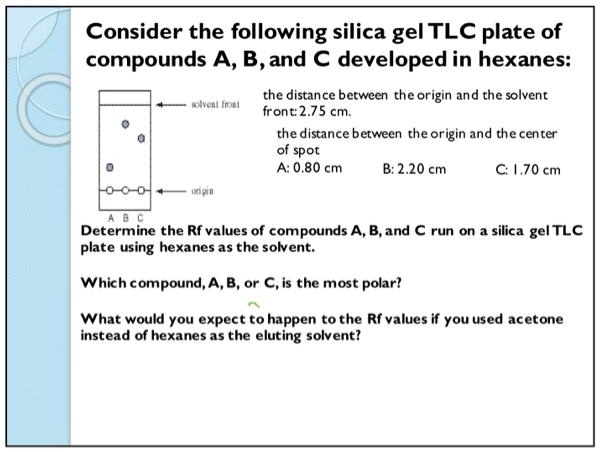
SOLVED: Consider the following silica gel TLC plate of compounds A, B,and C developed in hexanes: Een| Ine| the distance between theorigin and the solvent front:2.75 cm the distance between theorigin and

Preparation of mixed-mode stationary phase for separation of peptides and proteins in high performance liquid chromatography | Scientific Reports
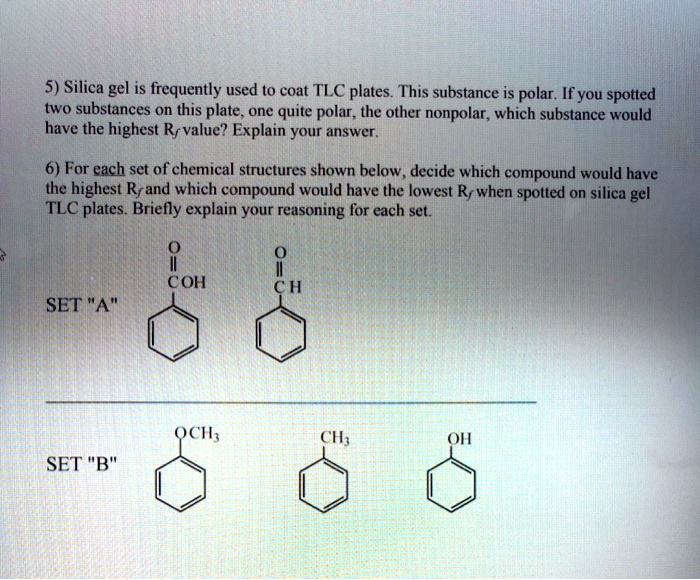
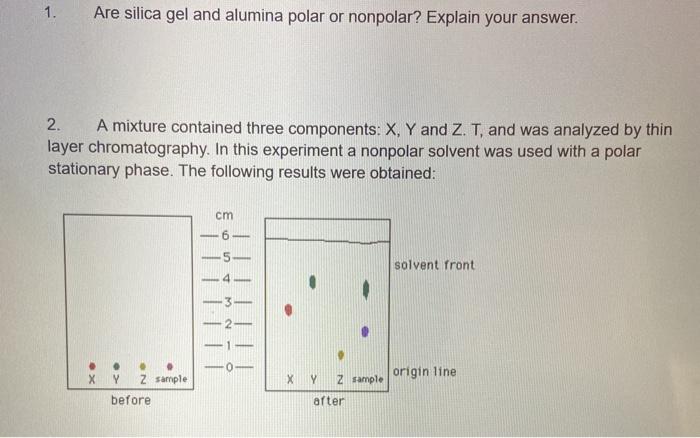
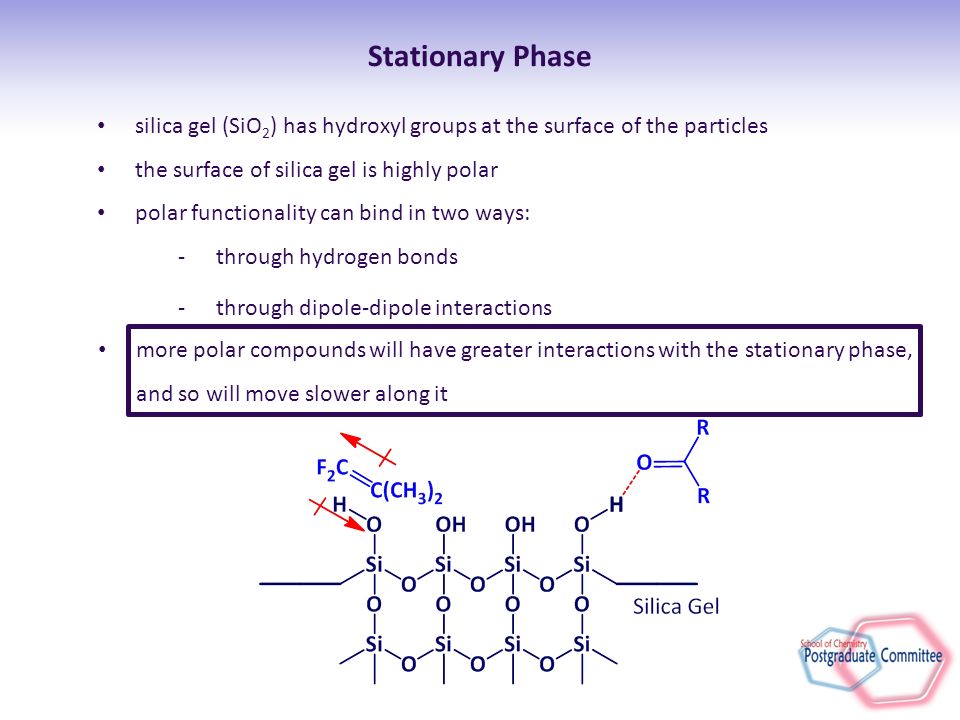
SOLVED: 5) Silica gel is frequently used to coat TLC plates. This substance is polar; If you spotted two substances on this plate one quite polar, the other nonpolar, which substance would





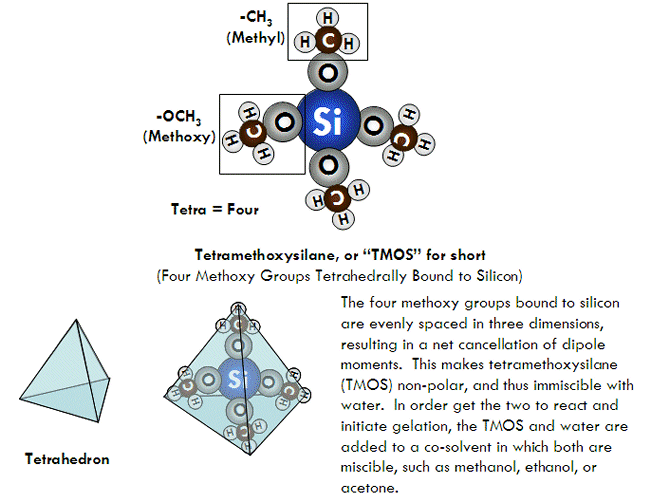
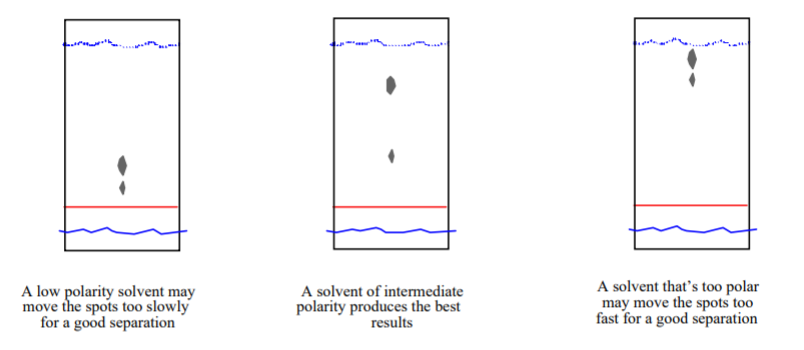


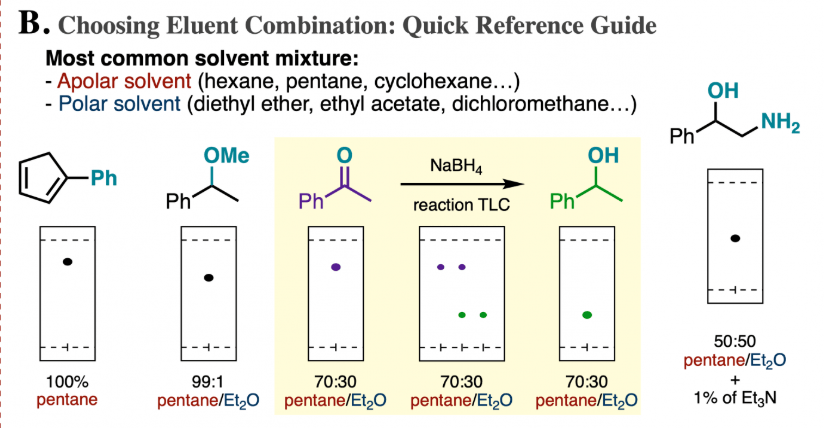
.png?revision=1&size=bestfit&width=1103&height=424)

